Digital Case Study – Three
Case history
32 year old man presented with a 6 month history of abdominal pain, diahorrea and rectal bleeding. Colonoscopic examination identified multiple areas of erythema in the caecum and ascending colon, which were biopsied and submitted for histopathology.
Microscopic findings
The biopsies of colonic mucosa showed intact glandular architecture. The lamina propria was moderately expanded by increased numbers of eosinophils, lymphocytes and plasma cells. No neutrophils and no granulomas were identified. Admixed with the inflammatory cells were collections (>15 cells) of plump oval to spindle shaped cells with smooth nuclear membranes, even chromatin and finely granular to cleared cytoplasm. A distinct pattern of concentric rimming around crypts by these cells was observed. There was diffuse staining for CD117 in these cells. CD2 immunohistochemistry was negative.
Biopsy figures
- Figures 1, 2 and 3 – colon.
- Figures 4 and 5 – distribution and cytomorphology of abnormal cells within mucosa.
- Figures 6 and 7 – CD117 immunostain
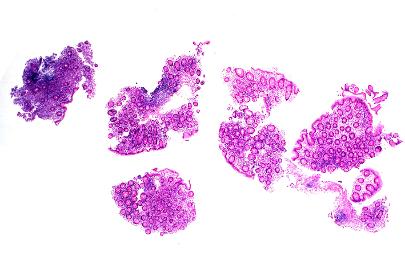
Figure 1 – colon
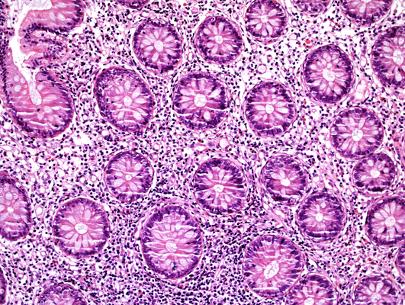
Figure 2 – colon
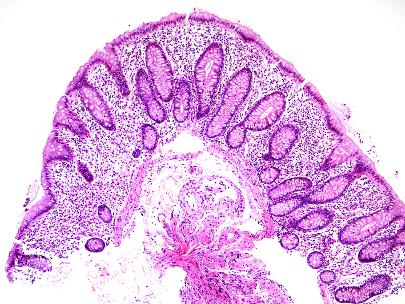
Figure 3 – colon

Figure 4 – abnormal cells in mucosa
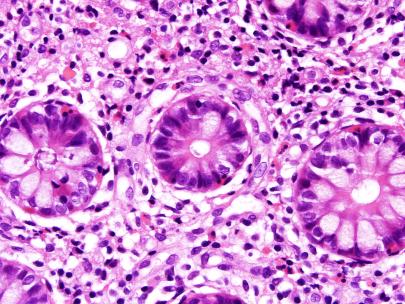
Figure 5 – abnormal cells in mucosa
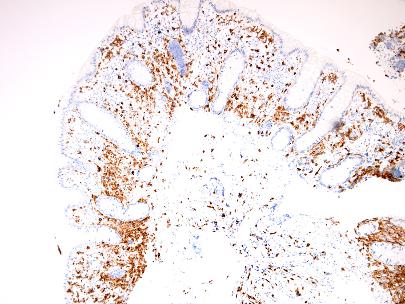
Figure 6 – CD117 IHC
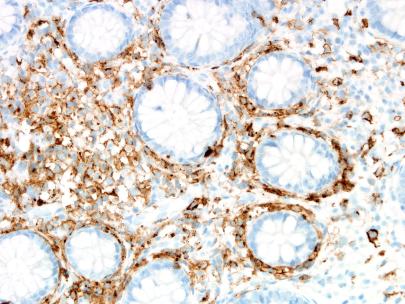
Figure 7 – CD117 IHC
Clinical follow-up
Clinical records revealed that the patient had previously presented with anaphylaxis to bee venom and recorded an elevated serum mast cell tryptase level of 195 ug/L. A subsequent bone marrow trephine confirmed marrow involvement by systemic mastocytosis.
Discussion
Mastocytosis is a clonal neoplastic proliferation of mast cells that can accumulate in any organ system. The current WHO classification system subtypes mastocytosis into:
- Cutaneous mastocytosis
- Indolent systemic mastocytosis
- Systemic Mastocytosis (SM) with an associated clonal haematological non-mast cell lineage disease
- Aggressive systemic mastocytosis
- Mast cell leukaemia
- Mast cell sarcoma
- Extracutaneous mastocytoma [1].
The diagnosis of SM can be made when major criteria and one minor criterion or at least three minor criteria are present [1]. The major and minor criteria are as follows:
Major criteria: multifocal dense infiltrates of mast cells (>15 mast cells in aggregates) detected in sections of bone marrow and/or other extracutaneous organ(s).
Minor criteria:
- In biopsy sections of bone marrow or other extracutaneous organs, >25% of the mast cells in the infiltrate are spindle-shaped or have atypical morphology or, of all mast cells in bone marrow aspirate smears, >25% are immature or atypical.
- Detection of an activating point mutation at codon 816 of KIT in bone marrow, blood or another extracutaneous organ.
- Mast cells in bone marrow, blood or other extracutaneous organs express CD2 and/or CD25 in addition to normal mast cell markers.
- Serum total tryptase persistently exceeds 20 ng/ml (unless there is an associated clonal myeloid disorder, in which case this parameter is not valid).
The presence of atypical mast cell infiltrates within gastrointestinal (GI) mucosal biopsies can be used to define SM according to WHO criteria and may be the first diagnosis in up to 67% cases [2]. Gastrointestinal involvement is common, with up to 92% of patients describing bloating, reflux, nausea, vomiting, abdominal pain, diarrhoea and weight loss [2-6]. These symptoms are thought to be due to direct infiltration of the mucosa by mast cells resulting in malabsorption and release of mast cell mediators such as histamines and leukotrienes.
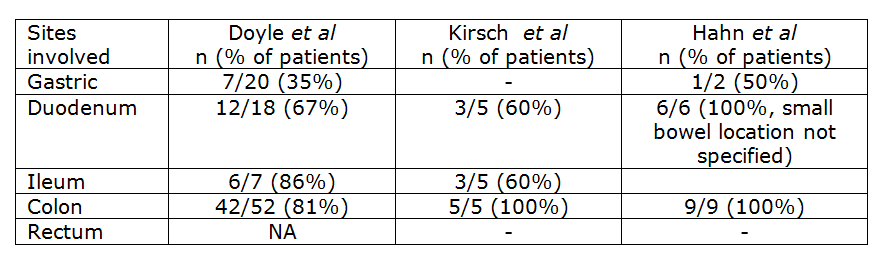
Although GI involvement is common in SM it is not necessarily a straight forward diagnosis as the histological changes and clinical presentation can mimic other disorders such as eosinophilic gastroenteritis and inflammatory bowel disease [4]. Endoscopic findings can also be subtle with 48% of patients described as having no abnormality in the study by Doyle et al [2] Documented endoscopic changes include erythema, congestion, erosions, loss of mucosal folds, granularity and nodularity [2,4,5]. Biopsies taken from abnormal mucosa are much more likely to show involvement by mastocytosis (93% of cases), however up to 63% of biopsies taken from apparently normal mucosa can also show the abnormal mast cell infiltrate [2]. Involvement of the gastrointestinal tract can also be patchy, therefore taking multiple biopsies from different sites and even apparently normal mucosa is optimal for diagnosis [6]. The stomach appears to be less commonly involved than small and large bowel as documented in the table below.
The WHO highlights four patterns of GI mucosal involvement by mastocytosis:
- Loosely scattered mast cells without dense aggregates but with an atypical immunophenotype and an activating point mutation of KIT usually in the setting of SM of some duration and involving the bone marrow.
- Slight increase in loosely scattered mast cells with occasional dense aggregates and an atypical immunophenotype with expression of CD25, usually associated with an activating point mutation in of KIT
- Diffuse compact infiltration of the mucosa by atypical mast cells, resembling the aggressive variant of SM in other tissues.
- Localised mast cell sarcoma.
Identifying atypical mast cells in the first scenario is likely to be achieved in the context of known SM. In the absence of a prior diagnosis and known clinical history, the histological picture most likely to be recognised is that of sheets and/or aggregates of mast cells in the lamina propria, often associated with a prominent infiltrate of eosinophils [2,5,6]. The aggregates are often subepithelial in distribution, although in the upper GI tract aggregates tend to be scattered throughout the lamina propria. Mast cells vary from rounded cells with moderate amounts of pale, eosinophilic cytoplasm to more commonly ovoid or spindled cells with more limited cytoplasm. Other histological findings such as architectural distortion, chronic duodenitis, villous blunting, lymphangiectasia and chronic gastritis have been described in association with mastocytosis and can suggest other disorders (such as medication/drug related injury and inflammatory bowel disease) or potentially obscure the disease [2,5,6]. Histological clues that assist in identifying a mast cell infiltrate include a rather characteristic pattern of mast cell rimming around crypts, subepithelial accentuation and the presence of plump spindle shaped cells in an overly ‘busy’ lamina propria.
Neoplastic mast cells show diffuse membranous staining with CD117 (KIT) and CD25/CD2 (co-expression of CD2 and/or CD25 is not observed in normal mast cells). A proportion of T cells can be positive for CD2 or CD25 and therefore careful correlation with morphology is required for correct interpretation (T cells have scant cytoplasm with strong staining for CD25 in contrast to mast cells, which often have more abundant cytoplasm and membranous staining for CD25). Mast cell tryptase, which is a highly reliable histochemical stain for identifying mast cells in other sites, is variable in GI biopsies with only patchy staining observed in 79% cases [2].
Mastocytosis is frequently associated with somatic activating point mutations within KIT. The most commonly observed mutation shows substitution of Val for Asp at codon 816 (D816V), which results in ligand independent activation of KIT-tyrosine kinase and provides relative resistance to the tyrosine kinase inhibitor imatinib.
The outcome of patients with mastocytosis is dependent on the subtype of disease; patients with aggressive disease including mast cell leukaemia may survive only a few months, whereas patients with indolent SM have a normal life expectancy. Gastrointestinal tract involvement is not in itself a marker of aggressive disease. Medical treatment includes mast cell stabilisers, proton pump inhibitors, and antihistamines. In advanced, aggressive or resistant disease, steroids and/or midostaurin, a multikinase inhibitor have been used with variable clinical response.
Inflammatory conditions of the bowel, which potentially mimic mastocytosis either clinically or histologically include irritable bowel syndrome (IBS), parasitic infection, eosinophilic gastroenteritis, inflammatory bowel disease and two more recently described ‘mast cell’ disorders: ‘mastocytic enterocolitis’ [7] and mast cell activation syndrome (MCAS) [8]. Patients with ‘mastocytic enterocolitis’ present with intractable diarrhoea, histologically normal endoscopic biopsies, ‘increased’ mast cells by immunohistochemistry and show clinical response to antihistamines or inhibitors of mast cell release. The study by Doyle et al reported overlapping mean mast cell counts per HPF for asymptomatic control patients, patients with IBS and mastocytic enterocolitis (19, 23 and 20/HPF respectively) [2]. The overlap in these numbers was considered by Doyle et al to be to great for clinical use and these authors postulated that patients with so called mastocytic enterocolitis had inappropriate mast cell activation such as that seen in MCAS. No significant increase in mast cells was reported by Hahn et al in patients with H pylori gastritis, bile reflux gastropathy, eosinophilic gastroenteritis, peptic duodenitis, coeliac disease, microscopic colitis and IBD compared to normal controls [5]. The main histological overlap is with cases of parasitic infection in which there is both an increase in mucosal eosinophils and mast cells [5].
In summary, patients with known SM are likely (70%) to have GIT involvement and a high histological index of suspicion should be maintained when reporting biopsies in this situation. Co-expression of CD117 (KIT) and CD2/CD25 is highly useful in identifying neoplastic mast cells. Clues to the diagnosis in patients without a history of SM include a ‘busy’ lamina propria, prominent eosinophils and aggregates of cells with pale, finely granular cytoplasm rimming crypts or in a subepithelial location. Parasitic infections should be considered as a histological differential diagnosis and pursued clinically when elevated numbers of eosinophils and mast cells is established.
References
- Swerdlow, SH.; Campo, E.; Harris, NL., et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008.
- Doyle LA, Sepehr GJ, Hamilton MJ, Akin C, Castells MC, Hornick JL.A clinicopathologic study of 24 cases of systemic mastocytosisinvolving the gastrointestinal tract and assessment of mucosalmast cell density in irritable bowel syndrome andasymptomatic patients. Am J Surg Pathol 2014;38:832–843.
- Jensen RT. Gastrointestinal abnormalities and involvement in systemic Mastocytosis. Hematol Oncol Clin North Am. 2000;14:579-623.
- Bedeir A, Jukic DM, Wang L et al. Systemic mastocytosis mimicking inflammatory bowel disease: a case report and discussion of gastrointestinal pathology in systemic Mastocytosis. Am J Surg Pathol 2006;30:1478-1482.
- Kirsch R, Geboes K, Shepherd NA et al. Systemic Mastocytosis involving the gastrointestinal tract: clinicopathologic and molecular study of five cases. Mod Pathol 2008;21:1508-1516.
- Hahn HP, Hornick JL. Immunoreactivity for CD25 in gastrointestinal mucosal mast cells is specific for systemic mastocytosis. Am J Surg Pathol 2007;31:1669-1676.
- Jakate S, Demeo M, John R, et al. Mastocytic enterocolitis: increased mucosal mast cells inchronic intractable diarrhea. Arch Pathol Lab Med. 2006; 130:362–367.
- Hamilton MJ, Hornick JL, Akin C, et al. Mast cell activation syndrome: a newly recognizeddisorder with systemic clinical manifestations. J Allergy Clin Immunol. 2011; 128:147–152.
